A clearer picture of an impermeable barrier
| 7 August, 2017 | Etienne Joly |
|

|
F1000Research author and F1000Prime Faculty Member Eitenne Joly discusses his area of research and his recent article the physical state of membranes in live cells

Membranes are far from inert seas of lipids
Membranes are an essential part of any living cell. The main architectural component of a biological membrane is the lipid bilayer, which constitutes an essentially impermeable barrier with the outside world. Transmission of signals and transport of components across biological membranes are carried out via the numerous proteins that are embedded in the membranes.
Over the past few decades, it has become apparent that membranes are far from being inert seas of lipids. Rather, they harbor a variety of microdomains, enriched with components, and endowed with different functions. To date, the mechanisms that govern the assembly of membrane microdomains remain poorly understood. There is, however, general consensus that membrane microdomains are sites of increased molecular order.
The trickiest problem about membrane microdomains (also known as rafts) is that they are both very labile and extremely small, i.e., less than 100 nanometers wide. This is much too small to be seen via standard optical microscopy, for which there is an inherent physical limit of about half a micron.
Lengthy acquisition times
For many years, scientists relied on biochemical extraction with certain detergents that were supposed to solubilize liquid-disordered non-rafts membranes, while liquid-ordered rafts would be resistant to solubilizing. This method is, however, seldom used nowadays because it is extremely crude and prone to artefacts.
Electron microscopy could be one solution to observe structures that are smaller than 100 nm, but this is not compatible with the observation of live cells. Instead, approaches based on super-resolution microscopy are becoming more popular, because the resolution limit of optical microscopy can be reduced to just a few tens of nanometers. Such approaches do, however, require elaborate equipment; are not always compatible with keeping cells alive and in physiological conditions; and often rely on unreasonably lengthy acquisition times.

Fluorescent lipid probes
Since 2004, I have been convinced that membrane microdomains correspond to areas where an initial seed comprised of proteins will prime particular membrane components to undergo a physical transition from a liquid state to a state more akin to a gel, or solid, or crystalline state.
Most membrane biophysicists tend to cringe at the idea of solid microdomains because they perceive that membranes must be in a liquid state for the cell to stay alive. This is, however, clearly not the case, and the existence of solid domains in biological membranes has been revealed both in bacteria, and more recently in yeast.
For many years, I’ve explored the technical means to discriminate liquid ordered from solid state in lipid bilayers. This is challenging because we want to work on live cells kept in physiological conditions, and on short timescales.
We used solvatochomic fluorescent lipid probes to explore the physical state of cell membranes, because the emission spectra vary depending on the physical properties of their environment. In this case, we used the C-laurdan probe for which the emission spectrum undergoes a red shift when there is a water molecule in the direct vicinity of the fluorochrome.
Once C-laurdan is inserted into a lipid bilayer, its emission spectrum discriminates between liquid disordered membranes, which are quite permeable to water, from liquid ordered and solid bilayers, which are much less permeable to water. This property has been used by many scientists to characterize the physical state of lipid bilayers in a whole variety of artificial membranes and cellular models.
Colour coding components
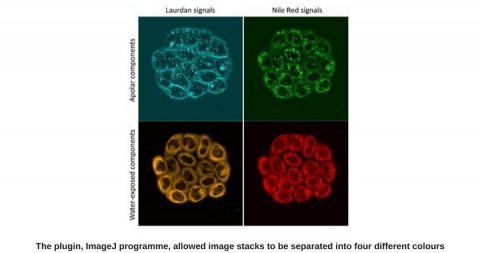
In 1990, the team of Enrico Gratton proposed using the numerical term GP (for generalized polarization) as a measure of the polarity of the Laurdan’s environment. Today, most scientists using sovatochromic probes, use GP to represent their data.
Calculating GP is based on the emission values at just two wavelengths, e.g. 440 and 490 nm for Laurdan-based probes. For the past decades, this was a convenient measure because most microscopes could then only acquire emissions at two or three wavelengths, using successive acquisitions through different filters.
What we did in our study
At the start of this study, we wanted to compare the properties of Laurdan with those of other lipid probes derived from it, namely M laurdan and C laurdan, to see if they would have more interesting properties and if we could discriminate lipid bilayers in liquid ordered state from those in solid state. We quickly found this was not the case, and confirmed the reports of other groups that C laurdan was a much better probe than laurdan to label the plasma membrane of live cells.
We realized we could make use of the spectral mode of the biphoton microscope we were using. In spectral mode, you can acquire whole emission spectra rather than just the two wavelengths at 440 and 490 nm. With those whole spectra, we could then carry out spectral unmixing, and effectively decompose the emission spectra into two components corresponding to the portion of the probe in water-exposed environments (i.e. in liquid disordered bilayers) and in hydrophobic environments (bilayers in liquid ordered and solid states).
This was a marked improvement over the GP calculations, but the results were still difficult to use to compare pictures. At that stage, we were lucky to benefit from the help of Farzad Fereidouni, University of California, who wrote a plugin for the ImageJ program. This tool represents the color-coded fraction of the first component and allowed us to optimize a whole series of parameters very efficiently, both for labelling the cells, as well as for image acquisition on the microscope, and analysis.
A liquid disordered state
With these new tools, we could define conditions and obtain highly reproducible results that hold true across a large selection of mammalian cell lines, as well as in cells from ectotherms, such as drosophila or amoebae. These results show that the lipid bilayers of intracellular compartments are dominantly in a liquid disordered state, while the plasma membrane is almost exclusively water-impermeable, corresponding to ordered states.
We now plan to use this approach to study cells in the context of various challenges such as temperature shifts or focal stimuli. In doing so, we hope to the able to document how events of signaling correlate with the physical state of the plasma membrane, and ultimately to understand how this correlates with the formation of membrane microdomains.

|


User comments must be in English, comprehensible and relevant to the post under discussion. We reserve the right to remove any comments that we consider to be inappropriate, offensive or otherwise in breach of the User Comment Terms and Conditions. Commenters must not use a comment for personal attacks.
Click here to post comment and indicate that you accept the Commenting Terms and Conditions.