F1000Trials: a new gateway to clinical trials
| 6 August, 2013 | Iain Hrynaszkiewicz |
|

|
Faculty of 1000 today launches the beta version of a new service, F1000Trials, which is currently freely available to all registered users. In this post we explain the need for this new publishing service, what the key features are, and who should use it. Read the press release here.
Are we at tipping point for clinical trial transparency?
In the last few months there has been unprecedented attention on the availability of information about clinical trials. Important initiatives in 2012-13 have focused on clinical trial results and trial registration, on clinical trial data, and on communicating the purpose and results of clinical trials in an understandable way to patients and clinicians. This final area is important but can be easily overlooked by publishing services that focus on primary research. But despite huge increases in the volume of research and literature, we also hear of failure to translate the billions spent on research into practice and policy. For medical research to have impact it needs to understood by clinicians and ultimately improve the care of their patients.
Randomized clinical trials are the gold standard for assessing the effectiveness of medical treatments, and systematic reviews – which draw together results of multiple trials – are the highest level of medical evidence and are embedded in health policy-making globally.
“We urgently need more transparency around clinical trial results, to avoid negative results being withheld. But we also need more engagement by clinicians and the public with all trials research. Tools like F1000Trials are an important part of that movement, away from the bad old days of trial results being entirely inaccessible, or – almost as bad – sitting in long technical documents, that busy clinicians may not have time to read.”
– Dr Ben Goldacre, researcher, and award-winning writer
It is too soon to say if these initiatives to increase the availability of all trial results will mean we are on the brink of a clinical trial information explosion. However, as randomized controlled trials and systematic reviews in healthcare have grown in their number and influence, there is an increasing need to identify the highest quality studies and the most important to clinical practice.
Introducing F1000Trials
F1000Trials provides clinicians and with a unique way to be alerted to new clinical trial articles and systematic reviews, with accompanying reviews and recommendations by F1000’s Faculty of experts.
F1000Trials is a continually updated, comprehensive database of randomized controlled trial articles (including early-phase oncology studies) and systematic review/meta-analysis articles drawn from over 300 key general and specialist medical journals. F1000 Faculty Members provide expert assessment of newly published articles, recommending the most noteworthy by assigning star ratings.
Features of F1000Trials
F1000Trials extends the widely recognized system of peer-nominated experts identifying great research in biology and medicine – around 3% of the literature – pioneered by F1000Prime. In this area (and era) of evidence-based medicine, our Faculty Members identified a need for comprehensive listing and coverage of all randomized controlled trials and systematic reviews – starting with the key general and specialist journals they read. We anticipate that approximately 5% of the reviewed articles will be tagged “Recommended” and awarded star ratings of Good (*), Very Good (**) or Exceptional (***).
Identification and consideration of negative trials and systematic reviews is essential for good medical evidence. Discussion of journals and databases of negative results have helped raise awareness of the importance of negative results (as has F1000Research), but identification of all published negative studies purely through bibliographic searching and key words is not possible. The F1000Trials editorial process adds tags to negative studies Faculty Members have read and reviewed, making them easy to find in the database.
Other features of F1000Trials include:
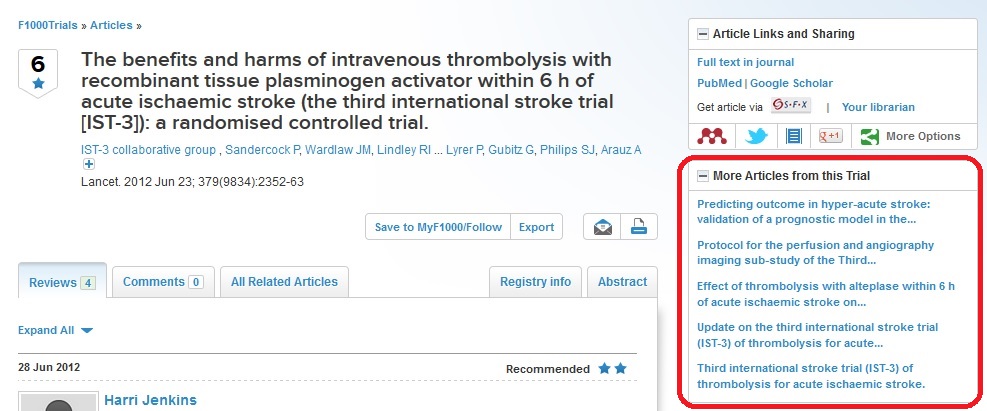
• A threaded publication trail – links, via the unique trial registry identifier, to trial registries and other PubMed-indexed articles about the same trial
• Powerful drug search engine that can identify generic and brand names from a single search
• Identification of trials that change clinical practice
F1000Trials also includes established features of F1000Prime, including email section alerts, article rankings, and hidden jewels, and social features such as the ability to follow Faculty Members.
Who should use F1000Trials?
F1000Trials is currently free to access to anyone who registers on the website, or is already registered with a Faculty of 1000 service. We encourage anyone interested in clinical trials and systematic reviews, or who has an interest in improving how we communicate information about clinical research, to try F1000Trials. We believe the service will be of particular interest to practicing clinicians, clinical researchers, health policy makers, health librarians, pharmacists, drug regulators, patient organizations and the pharmaceutical industry.
Speaking of the launch of F1000Trials, Prof. Peter Sandercock, University of Edinburgh and Principal Investigator on major clinical trials including the Third International Stroke Trial (IST-3), said: “I’m delighted that F1000Trials will, by linking and alerting us to new trial publications, contribute to greater transparency and efficiency in reporting and disseminating results of trials. This alerting mechanism will also facilitate prompt updating of systematic reviews of trials.”

|



I think this is a good trial because of its transparency.